Abstract
Objective: Circular RNAs (circRNAs) comprise a novel class of widespread non-coding RNAs that could regulate gene expression in eukaryotes. Glucocorticoid resistance is the primary factor of refractory relapse in patients with multiple myeloma (MM). This study was designed to reveal the role of circPVT1 in mediating glucocorticoid resistance in MM through regulating its target genes to inhibit apoptosis of MM cell and promote tumor stem cell survival.
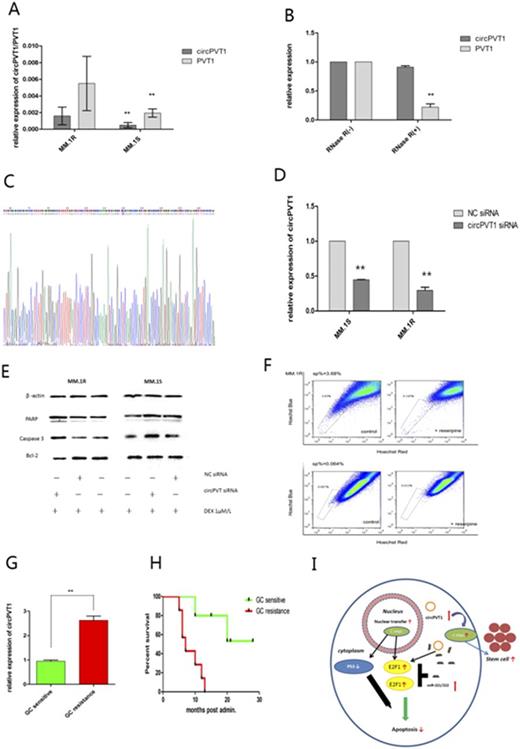
Methods and results: We determined with RT-PCR assay that circRNA PVT1 is differentially expressed between glucocorticoid resistant and sensitive MM cells (Figure A), and is closely correlated with prognosis (Figure G and H). We further investigated the role of circPVT1 in MM cell apoptosis and proliferation as well as its downstream signaling pathway mechanism. Silencing circPVT1 enhances sensitivity to glucocorticoid treatment (Figure D), promotes apoptosis and inhibits cell proliferation in resistant MM cell lines and myeloma cells in NOD-SCID mice, resulting up-regulation of the apoptosis pathway as measured by increased Caspase3, PARP and decreased Bcl-2 (Figure E). Measured by CCK-8 cell viability assay, ectopic expression of circPVT1 contributed to inducing proliferation and suppressing apoptosis in sensitive MM cell lines. Using Hoechst Staining, we found that MM stem cell population increases upon ectopic expression and decreases while silencing circPVT1, which revealed circPVT1 could facilitate the proliferation of stem cells to enhance glucocorticoid resistance (Figures F and I).
Conclusion: As a downstream of circPVT1, it promotes c-myc nuclear translocation, which intermediated by miRNAs to enhance its targets thus promotes the proliferation of stem cells and inhibits apoptosis. We concluded that circPVT1 may mediate MM glucocorticoid resistance and its downstream signaling pathway may represent a novel target for MM treatment.
Figure (A)RT-PCR showing the differential expression of circPVT1 and linear RNA PVT1 between MM.1R and MM.1S cell lines.** P <0.01. Error bars, S.D. (n=3). (B)RT-qPCR results showing the abundance of circPVT1 and PVT1 in MM.1R cells treated with RNase R. The levels of CircPVT1 and PVT1 were normalized to the values measured after mock treatments.** P <0.01. Error bars, S.D. (n=3). (C)The expression of circPVT1 was detected by RT-PCR and was validated by Sanger sequencing. (D)RT-PCR analysis of circPVT1 expression after treatment with siRNA targeting at the circPVT1.** P <0.01. Error bars, S.D. (n=3). (E)Western blot analysis of the levels of PARP, caspase 3, Bcl-2, control of β-actin in MM.1R and MM.1S transfected with circPVT1 siRNA or control siRNA, in absence of dexamethasone 1μM/L for 24 hours. (F)Using Hoechst Staining, analysis of side population of MM.1R transfected with circPVT1 siRNA or control siRNA. (G)RT-PCR showing the differential expression of circPVT1 of GC sensitive and resistant patients. ** P <0.01. Error bars, S.D. (n=28). (H)Kaplan-Meier analysis of the correlation between GC sensitivity and overall survival. (I)Diagram showing c-myc nuclear translocation facilitated by circPVT1, intermediated by miRNAs to enhance the targets of c-myc thus promotes the proliferation of stem cells and inhibits apoptosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal